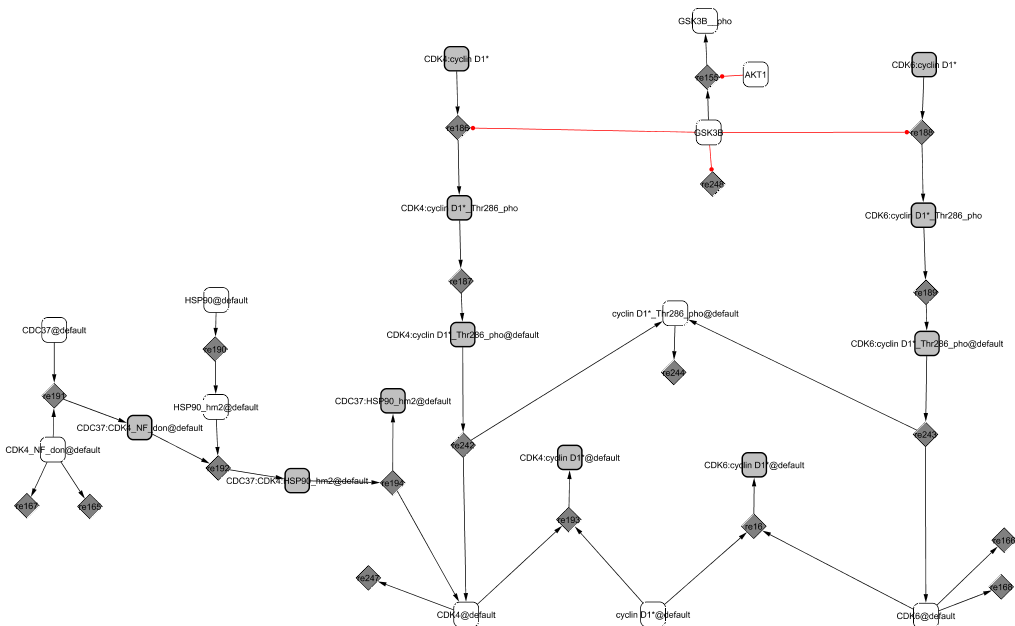
In early G1, cyclin D1 is in the cytoplasm where it binds to the modified forms of the cyclin-dependent kinases CDK4 and CDK6 (Sherr and Roberts, 1999). CDK4 and CDK6 (the latter is not explicitly shown in the diagram) are initially in their native form and cannot associate with their cyclin partner. They need to be properly folded by Hsp90, itself recruited by CDC37 (Roussel, 1999). Only then can the kinases and the cyclin associate into active complexes.
The CIP/KIP family members, p21CIP1 and p27KIP1, direct the accumulation of the complexes in the nucleus (Sherr and Roberts, 1999). Their role is not clear but they seem to also favour the tight association between the proteins. We will mention in the cyclin-dependent kinase inhibitor module (p21CIP1 / p27KIP1 module) their dual role as an activator of the cell cycle when in complex with CycD1/CDK4,6 and as an inhibitor when in complex with CycE1/CDK2 and CycA2/CDK2. When associated with CycD1/CDK4,6, the trimers allow the sequestration of the two inhibitors p21CIP1 and p27KIP1, preventing the association of the inhibitors with CycE1/CDK2 and CycA2/CDK2 too early during the cycle. In late G1, the kinase GSK3 phosphorylates cyclin D1 at Thr-286 (Coqueret, 2002). The phosphorylated complex moves to the cytoplasm where it dissociates and as a consequence, the cyclin gets degraded (Yu et al., 1998).
The complexes CycD1/CDK4 and CycD1/CDK6 act as sensors of growth factors. When activated, they precipitate the cells into S phase by further phosphorylating RB (Planas-Silva and Weinberg, 1997; Weinberg, 1995).
|

